Calculate Ph of Buffer After Adding Naoh
0003 mol 0 - E 00795 mol 0 00003 mol - HC 3H 5O 2. K b CH3CH2NH256104 The order of answers will be.

Calculate Ph Of Buffer After Adding Strong Base Youtube
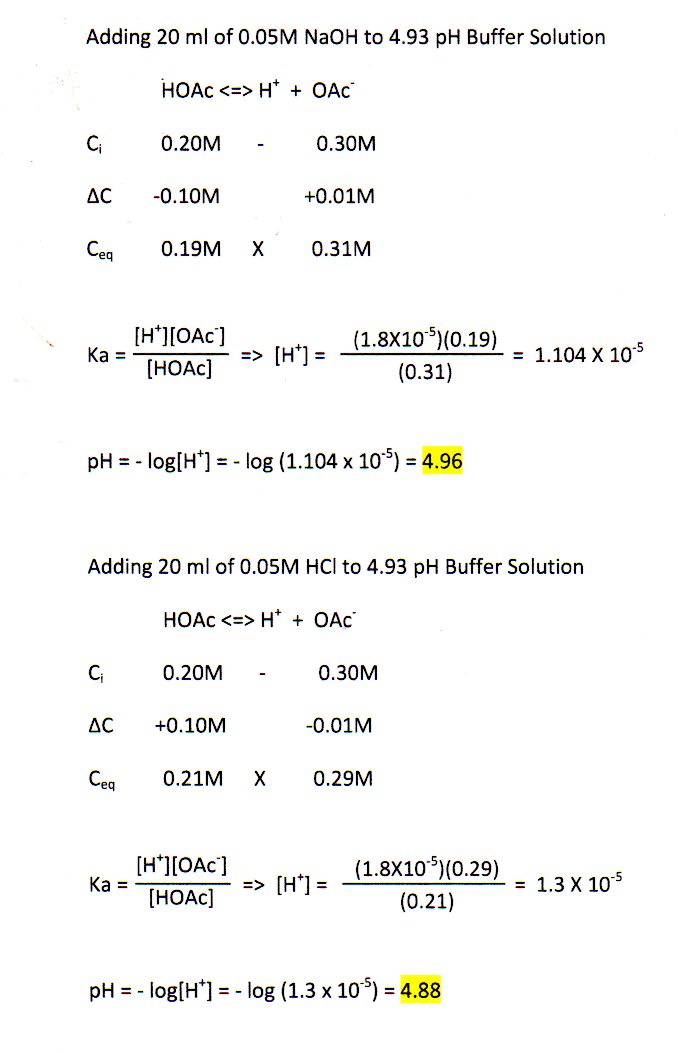
All the added OH-will react with HAc to give more Ac-.
. Adding HCl and NaOH to a buffer solution. Before the addition of NaOH the water is neutral and thus pH 7 After the addition of NaOH. NaOH 0020 molL 0020 M NaOH is a strong base dissociates 100 OH- 0020 M What will happen when we add the NaOH.
The pH of your NaOH solution is 13. The resulting solution contains a buffer. 4 pts Chemical equation and change table to calculate pH after adding 82 mL of 10 M NaOH.
C For comparison calculate the pH after 10 mL of 010 M NaOH is added to 100 mL of a solution of an unbuffered solution with a pH of 474. Show your work in detail for one of the volumes Add 15 mL of NaOH HC 3H 5O 2 0275 mol L 0030 L 000825 mol NaOH 0200 mol L 00015 L 00003 mol I 00825 mol 0. By how much will the pH of 01 M acetic buffer at pH575 change after addition of 00005 moles of NaOH.
PK a -logK a unitless pK a unitless pH moleL CA moleL CB This calculator is valid for a buffer of a weak acid and its conjugate base of the same system. Calculate the change in pH when 0001 mole of hydrochloric acid HCl is added to a liter of solution assuming that the volume increase upon adding the HCl is negligible. What is the pH after adding 100 mL of 001 M NaOH to 010 L of this bufferKa for NH4 if 570 x 10-10 arrow_forward 2500 mL of a saturated CaOH2 sample requires 2250 mL of 00250 M HCl to neutralize it.
Of moles of OH added 1 molL 101000 L 001 mol Volume of the solution 1 101000 L 101 L OH 001 mol 101 L 00099 M pOH -log OH -log 00099 2 pH pKw - pOH 14 - 2 12 Change in pH 12 - 7 5. The mol of base is added to the buffers base and the bases. For 3000 mL of a buffer solution that is 0325 M in CH3CH2NH2 and 0235 M in CH3CH2NH3Cl calculate the initial pH and the final pH after adding 0032 mol of NaOH.
In this video I will teach you how to calculate the new pH of a buffer solution after adding an acid. Clearly mark your final pH value for each one. This means that the pH of the solution will be equal to the pK_a of the weak acid because the concentrations of the weak acid and of the conjugate base are equal.
PH 638 1 738. We can use the given molarities in the Henderson-Hasselbalch Equation. This answer is the same one we got using the acid dissociation constant expression.
Calculating pH To calculate pH apply the formula pOH -log OH-. For each of the following solutions calculate the initial pH and the final pH after adding 0010 mol of NaOH. Calculating Changes in a Buffer Solution Example 1.
Calculate pH of a buffer prepared by adding 10mL of 010M acetic acid to 20mL of 01M sodium acetate. 5 pts Chemical equation and change table to calculate pH after adding 64 mL of 10 M HCI. To calculate the pH of a buffer solution when base is added the Henderson-Hasselbalch equation pH pKa log acidbase is used.
This means that youre in the buffer region ie. For a the Henderson-Hasselbalch equation cepH mathrmpK_a logceA-ceHA comes in handy. PH -log H.
That means we can write 42 And the final answer is - pH changed by 466-475-009 pH unit. A solution is 0050 M in acetic acid HC 2 H 3 O 2 and 0050 M NaC 2 H 3 O 2. This skill is useful when asked to calculate the chang.
Here we have used the Henderson-Hasselbalch to calculate the pH of buffer solution. While in many aspects this question is almost identical we cant start it that easily as pH is quite different from pK a. Keep track of changes with a table.
For example if a system contains both CH3COOH and CH3COONa then the pH of this buffer can be calculated. Beta-HA beta-A- This is known as the half-equivalence point. Because your molarities and volumes of the acid and its conjugate base are equal this indeed reduces to simply cepH.
A Calculate the pH of a 0500 L buffer solution composed of 0700 M formic acid HCOOH K a 177 x 10 4 and 0500 M sodium formate HCOONab Calculate the pH after adding 500 mL of a 100 M NaOH solution. Work out -log 01 1. 189 x 10 3 M x H pH 272 b Calculate the pH after the addition of 15 200 and 405 mL of the base.
PH900 -log 73000 914 log 02 914 -014 9. The amount of hydronium ion initially present in the solution is H 3 O 10 474 18 10 5 M mol H 3 O 0100 L 18 10 5 M 18 10 6 mol H 3 O. Because NaOH is a strong base it ionizes completely in water.
C For 2200 mL of a buffer solution that is 0285 M in CH3CH2NH2 and 0255 M in CH3CH2NH. 2 What is the new pH after 0020 mol of solid NaOH is dissolved in 10 L of the buffer solution. Science Chemistry QA Library Calculate the pH of buffer solution after adding 40ml of O1M titrant NaOH to 50ml 01M of analyte HOAC pKa-476.
Note that here CH3COOH CA and CH3COONa CB. PK a C H 3 C OOH 474 A 300 B 444 C 474 D 504 Hard Solution Verified by Toppr Correct option is D Millimoles of C H 3 C OOH 0110 10 Millimoles of C H 3 C OON a 0120 20 From Henderson Hasselbalch equation pH pK a. Clearly mark your final answer.
Therefore the pH of the buffer solution is 738. Compare this to the pH if the same amount. To isolate the pH work out 14 - 1 13.
This means 01 mol of it will dissociate into 01 mol of Na and OH-. Next apply the formula pH pOH 14.

Acid Base Equilibria And Solubility Equilibria Ppt Download

Calculate Ph Of Buffer After Adding Strong Base Youtube

Calculate The Ph Of Buffer Solution Composed Of 0 1m Weak Base Boh And 0 2m Of Its Salt Ba Kb 1 8 Xx 10 5 For The Weak Base

Homework How To Calculate The Ph Of A Propanoate Buffer After Addition Of Sodium Hydroxide Chemistry Stack Exchange

How To Calculate The Ph Of A Buffer Solution After Adding Acid Hcl Youtube

What Is The Ph Of The Resulting Solution When Equal Volumes Of 0 1 M Naoh And 0 01 M Hcl Are Mixed
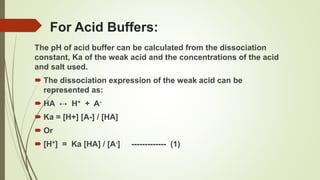
Polyprotic Acid Base Part 2 Buffers Youtube
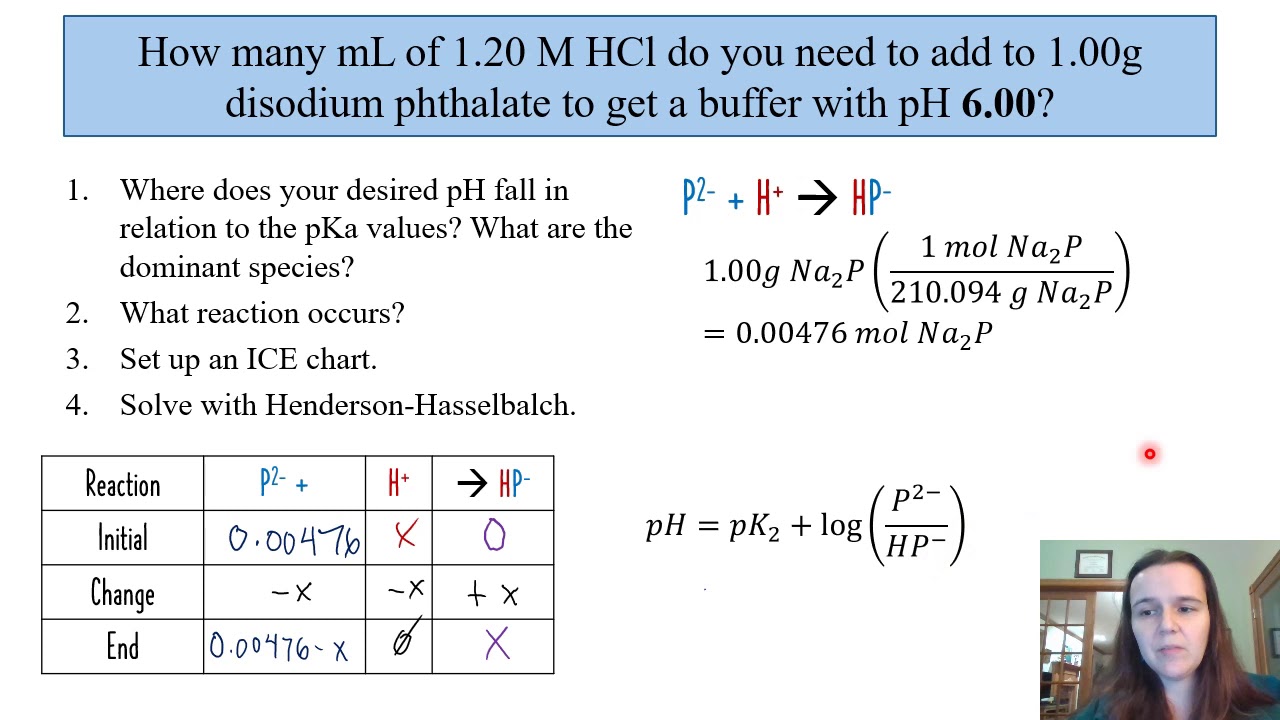
A Buffer Solution Contains 0 20 M Ch 3cooh And 0 30 M Ch 3coona At 25 Oc Ka 1 8 X10 5 What Is The Ph After The Addition Of A 20 0 Ml Of 0 050 M Naoh Or B

Buffer Solutions Video Khan Academy

Solved One Liter Of A Buffered Solution In Problem One Has Chegg Com
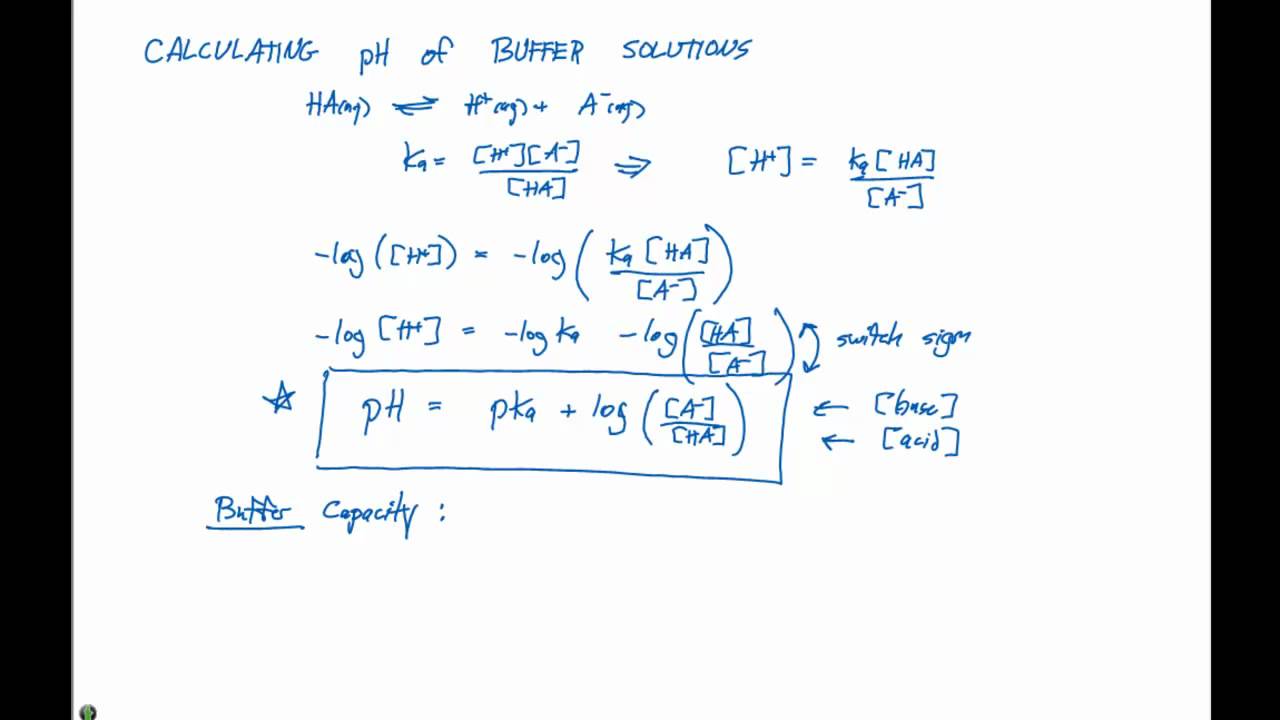
17 2 Calculating Ph Of Buffer Solutions Youtube

Ph And Pka Relationship For Buffers Video Khan Academy

Chapter 17 Acid Base Equilibria Part I 1dr Al Saadi Ppt Download

100 Ml Of 0 1 M Ch3cooh Is Mixed With 50 Ml Of 0 1 M Naoh Solution And Ph Of The Resulting Solution Is 5 The Change In Ph If 100 Ml Of




Comments
Post a Comment